A Comparison of Collagen Crosslink Content in Bone Specimens from Elective Total Hip Arthroplasty Patients with and without Type 2 Diabetes
Pritchard JM2, Papaioannou A1,3,4, Schwarcz HP5, Adachi JD3, DeBeer J6, Winemaker M6, Avram V6 and Willett T7
DOI10.4172/2469-6684.100028
Pritchard JM2*, Papaioannou A1,3,4, Schwarcz HP5, Adachi JD3, DeBeer J6, Winemaker M6, Avram V6 and Willett T7
1Geriatric Education and Research in Aging Sciences (GERAS) Centre, Hamilton Health Sciences, Hamilton, ON L8M 1W9, Canada
2Department of Interdisciplinary Science and Kinesiology, McMaster University, Hamilton, ON L8S 4L8, Canada
3Department of Medicine, McMaster University, Hamilton, ON L8S 4L8, Canada
4Department of Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, ON L8S 4L8, Canada
5School of Geography and Earth Sciences, McMaster University, Hamilton, ON L8S 4L8, Canada
6Department of Surgery, McMaster University, Hamilton, ON L8S 4L8, Canada
7Department of Systems Design Engineering, University of Waterloo, 200 University Avenue West, Waterloo, ON N2L 3G1, Canada
- *Corresponding Author:
- Pritchard JM
Department of Interdisciplinary Science and Kinesiology
McMaster University, 1280 Main Street West
Hamilton, ON, Canada.
Tel: 905-521-2100
E-mail: pritchar@hhsc.capritchar@hhsc.ca
Received date: September 13, 2016; Accepted date: September 22, 2016; Published date:September 26, 2016
Citation: Pritchard JM, Papaioannou A, Schwarcz HP, et al. A Comparison of Collagen Crosslink Content in Bone Specimens from Elective Total Hip Arthroplasty Patients with and without Type 2 Diabetes. J Bone Rep Recommendations. 2016, 2:3.
Abstract
Objective: To compare the amount of non-enzymatic and enzymatic collagen crosslinks in bone specimens from total hip replacement patients with and without type 2 diabetes (controls).
Methods: This ex vivo cross-sectional study included 34 bone specimens (13 from patients with type 2 diabetes, 21 from controls) from men and women ≥ 65 years. All participants were undergoing an elective total hip replacement due to osteoarthritis. Cancellers cores were extracted from the interior of the femoral neck/head and bone cores were reduced to approximately 50 mg of powder. High performance liquid chromatography (HPLC) was used to quantify pentosidine, pyridinoline (PYD) and deoxypyridoline (DPD), which were normalized to collagen content. The mean (SD) was calculated for continuous variables and an independent Student’s t-test was used to compare crosslink content between groups.
Results: 13 specimens were collected from participants with type 2 diabetes (mean [SD] age 73.8 [6.2] years) and 21 specimens were collected from controls (mean [SD] age 76.7 [6.8] years, p=0.222). There was no between-group difference in the amount of bone pentosidine (type 2 diabetes: 2.07 [0.94] mmol/mol collagen vs. control: 1.99 [0.60] mmol/mol collagen, p=0.753), PYD (type 2 diabetes: 219.2 [25.3] mmol/mol collagen vs. control: 208.1 [25.8] mmol/mol collagen, p=0.227) or DPD (type 2 diabetes: 132.3 [25.9] mmol/mol collagen vs. control: 130.0 [22.8] mmol/mol collagen, p=0.787).
Conclusion: Elective total hip replacement patients with and without type 2 diabetes have similar collagen crosslink profiles. Future studies should consider potential confounding factors, such as bone turnover rate, which may influence collagen crosslink content.
Keywords
Pentosidine; Advanced glycation end-products (AGEs); Type 2 diabetes; Bone; Collagen; Crosslinks
Introduction
Adults with type 2 diabetes are at an increased risk of hip fractures, which are associated with a reduction in quality of life and an increased risk of death [1]. The elevated fracture risk in adults with type 2 diabetes is paradoxical, as bone mineral density (BMD), a predictor of fracture in most adults, is higher compared to controls without type 2 diabetes [2]. However, other factors influence the propensity to fracture, including skeletal factors (i.e., structural and material properties of bone) and extra-skeletal factors (i.e., neuropathy, falls) [3].
Bone is principally made up of inorganic mineral and an organic component, which is predominantly type 1 collagen. Individual collagen molecules are linked together by the enzyme lysyl oxidase (LOX) forming covalent bonds between lysine and hydroxyl sine residues [4]. The formation of mature trivalent pyridinium crosslinks, including pyridinoline (PYD) and deoxypyridinoline (DPD), is necessary for optimal bone strength and toughness of bone [5]. In addition to the enzymatic crosslinks, non-enzymatic modification of collagen and other proteins occurs in the presence of glucose, a process that is enhanced with hyperglycemia. In the Millard reaction, glucose, its metabolic products and other sugars react with the amino groups of proteins, resulting in the production of advanced glycation end-products (AGEs) bound to protein [6]. In bone, the accumulation of a fluorescent AGE, pentosidine, is negatively related to ultimate strain, stress and fracture toughness of bone [7,8]. Other diabetes complications are associated with the accumulation of AGEs in tissues [9], and it is hypothesized that AGE accumulation in bone may help explain the elevated fracture risk in adults with type 2 diabetes. In addition, the glycation of type 1 collagen by AGE accumulation in bone may interfere with the formation of enzymatic crosslinks through competitive inhibition [10]. There may also be other factors that reduce the formation of enzymatic crosslinks in bone, including vitamin B6, homocysteine and oxidative stress. Vitamin B6, also known as pyridoxal-5′-phosphate, is a cofactor for LOX [11] and therefore required for the enzymatic crosslinking process. Vitamin B6 deficiency, a condition that may occur more often in patients with type 2 diabetes [12] is associated with a decrease in enzymatic crosslink content [13,14]. Patients with type 2 diabetes also have elevated serum homocysteine and biomarkers of oxidative stress [15]. Given that homocysteine and oxidative stress impair normal collagen crosslinking [16,17] these factors may be involved in altering the collagen crosslink profile, making diabetic bone more prone to fracture.
The objectives of this study were therefore to compare: 1) the amount of pentosidine, and 2) the amount of mature enzymatic pyridinium crosslinks (PYD and DPD) in bone specimens from elective total hip replacement patients with type 2 diabetes and without type 2 diabetes. We hypothesized that pentosidine content is higher and pyridimium cross-link content is lower in bone specimens from patients with type 2 diabetes compared to bone specimens from patients without type 2 diabetes.
Methods
Study participants and setting
This cross-sectional ex vivo study was conducted using bone specimens harvested from patients following elective unilateral total hip arthroplasty (replacement) at the Juravinski Hospital in Hamilton, Canada. This was a secondary analysis of bone specimens collected for a study designed to assess bone mineralization in adults with type 2 diabetes [18]. Men and women were ≥ 65 years and were undergoing total hip replacement due to end-stage osteoarthritis. Participants in the diabetes group had a diagnosis of type 2 diabetes for ≥ 5 years. Potential participants were excluded from the study if they: 1) Were currently taking or had taken Osteoporosis-related medication (bisphosphonates, hormone therapy, selective estrogen receptor modulator, calcitonin, parathyroid hormone or denosumab) in the past 24 months; 2) Had a history of metastatic cancer in the past 10 years; 3) Were currently taking systemic glucocorticoids for 3 months at a dose of >2.5 mg/day; and 4) Had a diagnosis of severe renal disease (creatinine clearance <30 ml/min), hyperparathyroidism, hypoparathyroidism, Paget's disease, Cushing's syndrome, or osteogenesis imperfecta. The Hamilton Integrated Research Ethics Board approved the study.
Bone specimen preparation
Following surgical removal, the proximal femurs were wrapped in saline soaked gauze and stored at -80° C for 18 months until further processing. The specimens were defrosted for 24 h before 0.5 cm diameter cancellous bone cores were extracted from the interior of the femoral neck/head. The cores were entirely cancellous bone and did not include subchondral or cortical bone affected by osteoarthritis. Only one core could not be obtained from the samples ascertained for the original study [18] due to degraded trabecular bone. The cores were degreased using chloroform and methanol, dehydrated in 70%, 80%, 90%, 96% and 100% ethanol baths and dried at 60°C for 4 h. After drying, the cores were reduced to a fine powder using a liquid nitrogen cooled mill (SpexPrep Model 6770 Freezer Mill, Spex Sample Prep, New Jersey, USA).
Quantification of amount of collagen and crosslinks
Mature pyridimium enzymatic and non-enzymatic crosslinks were quantified using established methodology with high performance liquid chromatography (HPLC) [19-21]. Briefly, approximately 50 mg of bone powder was hydrolyzed using 11 M HCl at 110°C for 24 h. The samples were added to a buffer solution comprised of 10% acetonitrile, 1% HFBA and water plus an internal standard (pyridoxine). Standards were used to quantify pentosidine (PolyPeptide Group, Strasbourg, France), PYD and DPD (Qiagen, Hilden, Germany). Collagen content was measured by quantifying hydroxyproline in the samples using hydroxyproline and amino acid standards (Sigma-Aldrich). The HPLC columns were Agilent Zorbax Eclipse XDB-C18 Reversed- Phase C18 HPLC columns (150×4.6 mm, 5 μm particle size, 80 Å pore size, end-capped; Agilent Technologies, Mississauga, ON, Canada). For each sample, an elution profile measuring fluorescence and elution time was obtained, and the areas under the peaks were measured and compared to a standard curve in order to calculate the concentration of pentosidine, PYD, DPD and hydroxyproline. Cross-link concentration was then normalized to collagen content (mmol crosslink/mol collagen).
Additional data collection
For descriptive purposes, the participant’s age, height, weight and medications were abstracted from the medical chart. Random, non-fasting blood glucose was ordered by the surgeon preoperatively and was also abstracted from the patient’s chart. The number of years since diabetes diagnosis was obtained by chart abstraction or participant self-report. The Physical Activity Scale for the Elderly (PASE) was administered to estimate participation in activities over the past 7 days [22] and the age-adjusted Charlson Index, a measure of comorbidity, was determined for each participant.
Statistical analysis
As all data were normally distributed, the mean (standard deviation [SD]) and frequency (percent, %) were calculated for descriptive variables and crosslink content. An independent Student’s t-test was used to compare means between groups or a χ2 tests was used to determine the difference in frequencies. We performed a sample size calculation using the findings from Wang and colleagues, who measured pentosidine content in human cadaver femurs from young (age 19-49 years), middleaged (age 50-69 years) and elderly donors (age >70 years) [7]. The investigators found a mean difference in bone pentosidine of 0.49 mmol/mol with an average SD of 0.26 mmol/mol using a power of 80% and α level of 0.05, we estimated a sample size of at least 5 participants per group. Analyses were completed using SPSS version 22.0 for Macintosh (IBM Corp., Markham, Canada). The criterion for statistical significance was α level <0.05.
Results
A total of 13 specimens from participants with type 2 diabetes and 21 specimens from controls were included. The descriptive characteristics are presented in (Table 1). The mean (SD) age, BMI, number of prescribed medications and PASE score were similar for participants with and without type 2 diabetes. Of the participants with type 2 diabetes taking medication, most participants (53.8%) were taking a biguanide (metformin). Two participants were taking two types of medication (insulin and biguanide, biguanide and insulin secretagogue sulfonylurea).
| Type 2 Diabetes n=13 |
Control n=21 |
p-value | |
|---|---|---|---|
| Age, years | 73.8 (6.2) | 76.7 (6.8) | 0.222 |
| % female, n (%) | 6 (46.1) | 14 (66.7) | 0.238 |
| BMI, kg/m2 | 30.1 (7.4) | 28.8 (5.5) | 0.576 |
| Use walking aid, n (%) | 8 (61.5) | 13 (61.9) | 0.784 |
| Number of prescription medications | 6.0 (2.9) | 5.0 (1.6) | 0.206 |
| Type of diabetes medication | |||
| Not taking medication, n (%) Insulin, n (%) Biguanide, n (%) Insulin secretagogue sulfonylurea, n (%) |
3 (23.1) 3 (23.1) 7 (53.8) 2 (15.4) |
||
| Number of years since diabetes diagnosis, years | 14.2 (7.3) | -- | -- |
| Random glucose, mmol/L | 8.5 (2.1) | 5.7 (1.1) | <0.001 |
| PASE score | 84.5 (39.7) | 78.7 (83.7) | 0.840 |
| Age-adjusted Charlson Index | 4.4 (0.9) | 2.3 (2.4) | 0.006 |
Table 1: Descriptive characteristics of study participants.
The mean (SD) number of years with a diagnosis of type 2 diabetes was 14 (7) years. Random blood glucose and the ageadjusted Charlson Index were higher in participants with type 2 diabetes compared to controls.
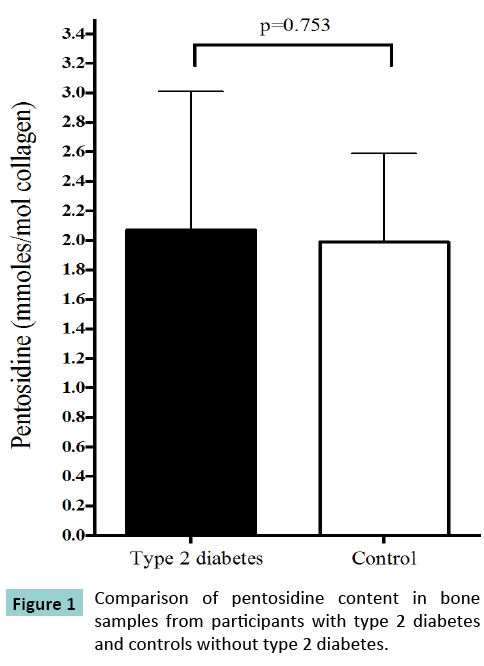
The comparison of collagen crosslink content from bone specimens from participants with and without type 2 diabetes is displayed in (Figure 1). There was no between-group difference in the mean (SD) amount of bone pentosidine (2.07 [0.94] mmol/ mol collagen vs. 1.99 [0.60] mmol/mol collagen, p=0.753), PYD (219.2 [25.3] mmol/mol collagen vs. 208.1 [25.8] mmol/mol collagen, p=0.227) or DPD (132.3 [25.9] mmol/mol collagen vs. 130.0 [22.8] mmol/mol collagen, p=0.787) between participants with and without type 2 diabetes, respectively.
Discussion
This study did not reveal higher levels of pentosidine in bone specimens from participants with type 2 diabetes compared to controls without diabetes. In addition, there was no betweengroup difference in the amount of mature pyridinium enzymatic crosslinks, PYD and DPD. These findings are not consistent with the hypothesis that elevated bone pentosidine and suppressed bone enzymatic crosslinks are responsible for diabetic bone fragility.
Various studies have explored the role of pentosidine in diabetic bone fragility, and most studies have shown that bone pentosidine levels are higher in rodent models of type 2 diabetes [14,23], leading to reduced stiffness, elastic modulus and energy absorption of bone from diabetic rats [14]. In humans with type 2 diabetes, urinary pentosidine levels are associated with a higher risk of incident osteoporotic fracture individuals with type 2 diabetes [24] and increased serum pentosidine is associated with prevalent vertebral fractures in women with type 2 diabetes, independent of potential confounding factors [25]. However, these studies in humans are limited, as the relationship between plasma pentosidine and cortical bone pentosidine is modest at best (r=0.25) [26], and given that pentosidine accumulates in other tissues, it is likely that systemic pentosidine is not reflective solely of bone pentosidine accumulation. In addition, serum, urine and bone pentosidine appear to be influenced by bone turnover rate. Schwartz and colleagues reported a positive relationship between urine pentosidine and serum procollagen type 1 N-terminal propeptide (s-P1NP) and C-terminal crosslinking telopeptide of type 1 collagen (s-CTX) [24]. In addition, Hein and colleagues reported higher levels of serum pentosidine in patients with osteoporosis and higher bone turnover compared to patients with osteoporosis and lower or normal bone turnover [27]. These results suggest that as bone is turned over, bone pentosidine is released into circulation with traditional bone turnover markers. Similar findings have been reported regarding bone pentosidine. In dogs with lower bone turnover due to bisphosphonate treatment, bone pentosidine levels are higher [28]. Although not assessed in this study, bone turnover rate is an important factor to consider when examining systemic and in-situ bone pentosidine content.
The bone pentosidine content quantified in this study is similar to bone pentosidine content reported in other studies. Hernandez et al. [29] reported bone pentosidine levels of 2.25 (0.86-5.18) mmol/mol collagen from trabecular bone samples harvested from cadavers. Only one other study has investigated bone pentosidine levels in humans with and without diabetes. In contrast to our results, Oren and colleagues found 32% higher levels of bone pentosidine in 10 male total knee arthroplasty patients with type 2 diabetes compared to 10 controls without diabetes [30]. However, this study differed from our study in a few ways. First, the participants in the study by Oren et al. were approximately 10 years younger than the participants in our study. As AGEs have shown to accumulate in bone with age [31], it is possible that a ceiling effect was responsible for the lack of difference in bone pentosidine levels in our study. Second, the bone specimens from our study were primarily cancellous, and it is unclear whether the samples from the study by Oren et al. were cancellous or cortical. If the accumulation of AGEs is greater in cortical bone [26], the difference in pentosidine levels may be more pronounced in cortical bone compared to cancellous bone due to less bone turnover in cortical bone.
The secondary objective of this study was to examine the levels of enzymatic crosslinks in bone samples from patients with and without type 2 diabetes. It is well known that enzymatic crosslinks contribute to overall bone strength and fracture resistance [5], and therefore disrupting the enzymatic collagen crosslinking process could play a role in diabetic bone fragility. However, this has not yet been demonstrated in the literature, and our study also did not reveal a difference in mature enzymatic crosslinks in samples from patients with type 2 diabetes compared to patients without diabetes. In spontaneously diabetic WBN/Kob rats, Saito and colleagues reported similar levels of mature enzymatic crosslinks (PYD and DPD) from bone compared to levels found in control Wistar rats [14]. In humans, Oren et al. also observed no difference in the enzymatic crosslinks, PYD and DPD in bone samples in patients with diabetes compared to controls [30]. It may be that our participants with type 2 diabetes were too heterogeneous and experienced different bone turnover rates due to different disease durations, glycemic control or diabetes medications.
There are study limitations to acknowledge. This was a secondary study and the sample size was based on the primary outcome for another study [18]. However, a similar sample size was used in the study by Oren and colleagues (10 participants without diabetes and 10 participants with diabetes) [30]. Also, serum biochemistry was not captured in this study, and there are various potentially confounding factors that may interfere with collagen crosslinking, such as bone turnover markers (s-P1NP and s-CTX), vitamin B6, homocysteine and markers of oxidative stress. While the relationship between homocysteine and hip osteoarthritis is not known, higher levels of homocysteine are reported in many other age-related chronic diseases, such as cardiovascular diseases [32]. It may be important to consider factors like serum homocysteine, markers of oxidative stress, vitamin B6 and other confounding diseases, such as cardiovascular diseases, when exploring the effect of type 2 diabetes on the collagen crosslink profiles in bone. While some studies report suppressed bone turnover in patients with type 2 diabetes [33], the absence of bone turnover markers in this study is a limitation, as bone turnover could be influenced by type 2 diabetes disease duration, severity and anti-hyperglycemic medication, and bone turnover may influence crosslink content. While random blood glucose was measured pre-operatively, other superior measures of longer-term glycemic control, such as HbA1c, were not measured. Finally, pentosidine was the only non-enzymatic crosslink assessed in this study, and given that pentosidine makes up approximately 1% of total fluorescent AGEs [34], future studies should investigate whether there is a difference in other fluorescent AGEs in bone from patients with type 2 diabetes.
Conclusion
In conclusion, this study revealed that there is no difference in bone pentosidine content or enzymatic crosslink content in participants with type 2 diabetes compared to controls without diabetes. In order to rule out the involvement of an altered crosslink profile in diabetic bone fragility, potentially confounding factors such as bone turnover, vitamin B6, homocysteine, markers of oxidative stress, type 2 diabetes duration and severity and medication use should be considered in future studies.
Acknowledgements
We would like to thank Vezna Relic, Hayley McCormack and Michelle Ball who assisted in participant recruitment. We are grateful for the cooperation and assistance from all clinical staff in the Juravinski Hospital Operating Room, and Martin Knyf for his technical expertise with sample preparation. Postdoctoral funding was provided by Hamilton Health Sciences Foundation and other funding by an unrestricted grant from Amgen Canada.
References
- Janghorbani M, Van Dam RM, Willett WC, Hu FB (2007) Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am JEpidemiol 166:495-505.
- Ma L, Oei L, Jiang L, Estrada K, Chen H, Wang Z, et al. (2012) Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol 27: 19-32.
- Felsenberg D, Boonen S (2005) The bone quality framework: determinants of bone strength and their interrelationships, and implications for osteoporosis management. ClinTher 27:1-11.
- Robins SP, Bailey AJ (1977) The chemistry of the collagen cross-links. Characterization of the products of reduction of skin, tendon and bone with sodium cyanoborohydride. Biochem J 163:339-346.
- Oxlund H, Barckman M, Ortoft G, Andreassen TT (1995) Reduced concentrations of collagen cross-links are associated with reduced strength of bone. Bone 17: 365-371.
- Grandhee SK, Monnier VM (1991) Mechanism of formation of the Maillard protein cross-linkpentosidine. Glucose, fructose, and ascorbate as pentosidineprecursors. J BiolChem 266:11649-11653.
- Wang X, Shen X, Li X, Agrawal CM (2002) Age-related changes in the collagen network and toughness of bone. Bone 31:1-7.
- Karim L, Vashishth D (2012) Heterogeneous glycation of cancellous bone and its association with bone quality and fragility. PLoS ONE 7: 35047.
- Sell DR, Lapolla A, Odetti P, Fogarty J, Monnier VM (1992) Pentosidine formation in skin correlates with severity of complications in individuals with long-standing IDDM. Diabetes 41:1286-1292.
- Saito M, Fujii K, Soshi S, Tanaka T (2006) Reductions in degree of mineralization and enzymatic collagen cross-links and increases in glycation-induced pentosidine in the femoral neck cortex in cases of femoral neck fracture. OsteoporosInt 17:986-995.
- Bird TA, Levene CI (1982) Lysyl oxidase: evidence that pyridoxal phosphate is a cofactor. BiochemBiophys Res Commun 108:1172-1180.
- Nix WA, Zirwes R, Bangert V, Kaiser RP, Schilling M, Hostalek U, Obeid R (2015) Vitamin B status in patients with type 2 diabetes mellitus with and without incipient nephropathy. Diabetes Res ClinPract 107:157-165.
- Fujii K, Kajiwara T, Kurosu H (1979) Effect of vitamin B6 deficiency on the crosslink formation of collagen. FEBS Lett 97:193-195.
- Saito M, Fujii K, Mori Y, Marumo K (2006) Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. OsteoporosInt 17:1514-1523.
- Dominguez LJ, Galioto A, Pineo A, Ferlisi A, Ciaccio M, et al. (2010) Age, homocysteine, and oxidative stress: relation to hypertension and type 2 diabetes mellitus. J Am CollNutr 29:1-6.
- Raposo B, Rodriguez C, Martinez-Gonzalez J, Badimon L (2004) High levels of homocysteineinhibitlysyl oxidase (LOX) and downregulate LOX expression in vascular endothelial cells. Atherosclerosis 177: 1-8.
- Nojiri H, Saita Y, Morikawa D, Kobayashi K, Tsuda C (2011) Cytoplasmic superoxide causes bone fragility owing to low-turnover osteoporosis and impaired collagen cross-linking. J Bone Miner Res 26:2682-2694.
- Pritchard JM, Papaioannou A, Tomowich C, Giangregorio LM, Atkinson SA (2013) Bone mineralization is elevated and less heterogeneous in adults with type 2 diabetes and osteoarthritis compared to controls with osteoarthritis alone. Bone 54:76-82.
- Willett TL, Sutty S, Gaspar A, Avery N, Grynpas M (2013) In vitro non-enzymatic ribation reduces post-yield strain accommodation in cortical bone. Bone 52:611-622.
- Bank RA, Beekman B, Verzijl N, de Roos JA, Sakkee AN, et al. (1997) Sensitive fluorimetric quantitation of pyridinium and pentosidine crosslinks in biological samples in a single high-performance liquid chromatographic run. J Chromatogr B Biomed SciAppl 703:37-44.
- Burton B, Gaspar A, Josey D, Tupy J, Grynpas MD, et al. (2014) Bone embrittlement and collagen modifications due to high-dose gamma-irradiation sterilization. Bone 61:71-81.
- Washburn RA, Smith KW, Jette AM, Janney CA (1993) The Physical Activity Scale for the Elderly (PASE): development and evaluation. Journal of Clinical Epidemiology 46:153-162.
- Tomasek JJ, Meyers SW, Basinger JB, Green DT, et al. (1994) Diabetic and age-related enhancement of collagen-linked fluorescence in cortical bones of rats. Life Sci 55:855-861.
- Schwartz AV, Garnero P, Hillier TA, Sellmeyer DE, Strotmeyer ES (2009) Pentosidine and increased fracture risk in older adults with type 2 diabetes. J ClinEndocrinolMetab 94:2380-2386.
- Yamamoto M, Yamaguchi T, Yamauchi M, Yano S, Sugimoto T (2008) Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J ClinEndocrinolMetab93:1013-1019.
- Odetti P, Rossi S, Monacelli F, Poggi A, Cirnigliaro M (2005) Advanced glycation end products and bone loss during aging. Ann N Y AcadSci 1043:710-717.
- Hein G, Wiegand R, Lehmann G, Stein G, Franke S (2003) Advanced glycation end-products pentosidine and N epsilon-carboxymethyllysine are elevated in serum of patients with osteoporosis. Rheumatology 42:1242-1246.
- Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D (2009) Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. OsteoporosInt 20:887-894.
- Hernandez CJ, Tang SY, Baumbach BM, Hwu PB, Sakkee AN (2005) Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone 37:825-832.
- Oren TW, Botolin S, Williams A, Bucknell A, King KB (2011) Arthroplasty in veterans: analysis of cartilage, bone, serum, and synovial fluid reveals differences and similarities in osteoarthritis with and without comorbid diabetes. J Rehabil Res Dev 48:1195-1210.
- Nyman JS, Roy A, Tyler JH, Acuna RL, Gayle HJ (2007) Age-related factors affecting the postyield energy dissipation of human cortical bone. J Orthop Res 25:646-655.
- Kuo HK, Sorond FA, Chen JH, Hashmi A, Milberg WP (2005) The role of homocysteine in multisystem age-related problems: a systematic review. J Gerontol A BiolSci Med Sci 60:1190-1201.
- Gerdhem P IA, Akesson K, Obrant KJ (2005) Increased bone density and decreased bone turnover, but no evident alteration of fracture susceptibility in elderly women with diabetes mellitus. OsteoporosInt 16:1506-1512.
- Dyer DG, Blackledge JA, Thorpe SR, Baynes JW (1991) Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J BiolChem 266:11654-11660.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences